New Food Allergy Therapies Have Arrived – the FARE Summit 2024 had lots to share
This year’s Food Allergy Research & Education (FARE) Summit in Washington, D.C., featured a fresh lineup of sessions and events tailored to those affected by food allergies. Attendees explored cutting-edge developments and advancements in newly approved allergy therapies like Xolair and Neffy, plus there were opportunities to participate in workshops on effective advocacy, hear speakers share personal stories about managing food allergies and intolerances – and teen-specific sessions offered a supportive space for younger participants to connect.
There were countless additional networking opportunities to foster connections throughout the allergy community. I was finally able to spend time with many people I’ve ‘virtually met’ here online and on socials – and I thoroughly value all of the friendships I’ve made with all of you on this journey alongside our family.
Here’s an initial overview of the Xolair and Neffy sessions I attended and the takeaways I found most impactful, from my perspective* as a mom, as I gather information for our family.
Xolair for Food Allergies: A Promising Preventative Treatment
Image 1: Xolair warnings
One of the Summit’s most anticipated topics was about Xolair (omalizumab), an injectable biologic medication, already used for asthma and chronic hives, now recently approved for food allergy management (for ages 1 and up). Xolair offers a preventative approach, aiming to reduce the frequency and severity of allergic reactions by blocking immunoglobulin (IgE). However, it’s not without its complexities. I learned that a severe reaction can occur when you receive Xolair injections, especially when you begin treatment. In particular, it’s recommended the initial three doses require a two-hour observation period in a medical office. (see image 1)
Image 2: Xolair possible side effects
Not unlike any medication, there are possible side effects, including serious but rare risks like parasitic infections, heart and circulation problems, cancer (4 in 1,000 cases), and others, as well as more common side effects such as fever, muscle aches, and injection site reactions. (see image 2) Of major importance to also understand is that Xolair does not allow you to eat your allergens, you must continue to avoid all foods to which you are allergic.
Another key consideration with Xolair is its duration in the body—it typically takes about 16-20 weeks to reach steady-state levels in the body, so its effects aren’t immediate, and it can take up to a year for the medication to fully clear out of the body if you choose to stop treatment. This means patients cannot undertake any oral food challenges (OFC) until a full year after the last dose, making it crucial to complete any OFCs before beginning Xolair. Additionally, the Xolair dose for treatment of food allergy is significantly higher than for treatment of asthma or hives, which is important to note.
The discussion about Xolair at the Summit underscored that while Xolair presents exciting possibilities, it’s essential to weigh its benefits and risks carefully. Talk to your medical professionals to evaluate if Xolair is right for you or your family. (Important to note, if you’re taking or considering taking Dupixent [another biologic for eosinophilic disease, asthma, eczema, etc.] in addition to Xolair, read more here about what Dr. Robert A. Wood shared about Xolair and Dupixent – hint, it wasn’t especially positive or encouraging.)
Neffy: A New Type of Epinephrine Option
Image 3: Meet Neffy
Neffy, a new epinephrine nasal spray, was another major focus. Designed for people with type 1 allergies, both adults and children who weigh at least 30kg (66lb.), Neffy 2mg has several innovative features: it’s compact, needle-free, and easy to use—addressing concerns many people have about traditional auto-injectors. (see image 3) It’s recommended to carry two Neffy in case of severe reactions. Manufactured in Lakewood, NJ, Neffy is produced by the same facilities that manufacture Narcan and Valtoco, an epilepsy medication, underscoring confidence in its quality and reliability.
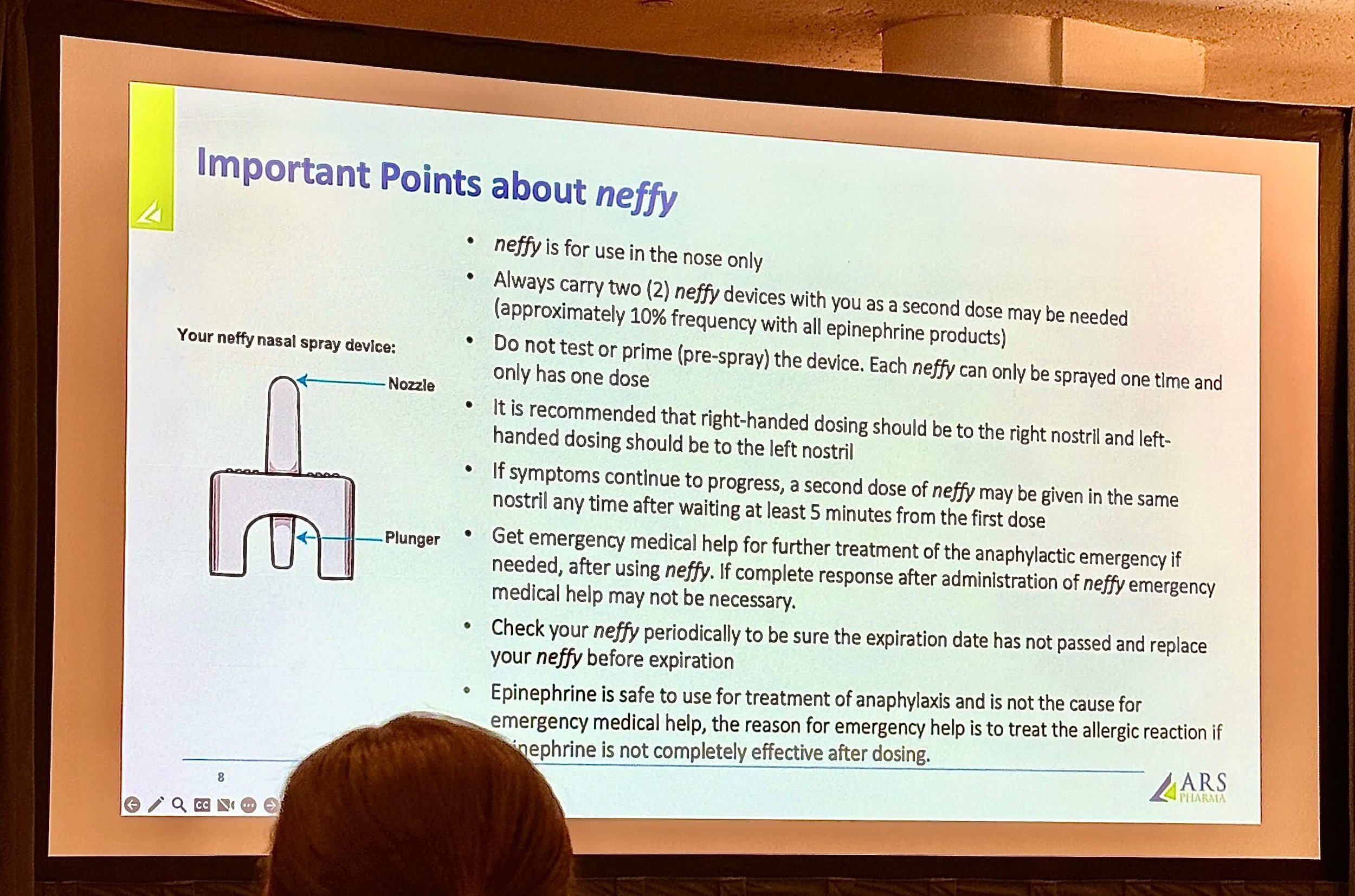
Image 4: Important Points about Neffy
Neffy is generally well tolerated, but it does have potential side effects, including mild headache and nasal discomfort. With the recent FDA approval, requirements mandate that it be dispensed with clear usage instructions, which fit conveniently in a carry case with two Neffy (order a carry case here at no cost, I did). See image 4 and this leaflet for more details on how to administer Neffy correctly, like understanding there’s no need to ‘prime’ the Neffy device; each Neffy only dispenses one dose and can only be sprayed once. It’s also important to know that each Neffy prescription does not come with a trainer device, unlike most auto-injectors. (I personally really wish it came with a trainer.)
Another important aspect to note is relative to cost, as with any new medications come cost considerations; currently, insurers may not cover Neffy. Though platforms accessible here offer discount options and how to obtain a prescription.
A question I raised was relative to the 2mg dose of Neffy, which is much higher than the standard 0.3 mg dose for people weighing at least 30kg (66lb.) in autoinjectors like EpiPen and Auvi-Q. This difference, according to experts, is due to the nasal delivery method which requires a higher dose to ensure sufficient administration of epinephrine. From what I understand, with the mist that Neffy administers, there is going to be some percent of loss in the mist as it is dispensed into the nose – some mist will not get absorbed in the nasal tissue, hence the higher 2mg dose.
Additional important Neffy info:
The shelf life for Neffy is 30-months, at room temperature
Neffy can maintain effectiveness up to 3 months at high temperatures (122 degrees F)
If accidentally frozen, Neffy can be thawed and maintain effectiveness (for how long, was not mentioned)
Neffy approval for children who weigh between 15 to 30kg has been submitted for approval (anticipated Summer 2025).
As with any prescription, be sure to discuss the risks and benefits of Neffy with your professional medical team. I have to say I am intrigued with the potential for our soon-to-be college aged son to have an option to ensure he always has epinephrine with him at all times. Neffy makes the ease of carrying epinephrine possible – it’s the thought of effectiveness and real world use that may have me err on the side of caution, and probably carry both traditional epinephrine auto-injectors along with Neffy for a while. It’s definitely promising.
Xolair and Neffy: food allergy therapy progress
The FARE Summit provided a wealth of information on the latest advancements in food allergy therapies, including Xolair and Neffy, which show promising potential in allergy management. While these treatments open up exciting possibilities, it's essential to carefully weigh the benefits and risks with your healthcare providers. I find myself sitting on the sidelines and not an early-adopter with new therapies like Xolair – probably because of my son’s history of failed oral immunotherapy and an EoE diagnosis (though I have several friends with food allergic teens, but not with EoE, who are definitely pursuing asap).
I do think we’ll pursue a Neffy prescription and carry multiple means of epinephrine administration as this new device becomes more mainstream with the food allergic community. You have to do what feels right for you and your unique circumstance – we’re all at different stages of this journey. I’m here for all of it, any questions or personal experiences with either Xolair, Neffy, food allergies or EoE, as always I’m here, please reach out.
* I am not a medical professional, all information and advice found on this website is not to take the place of professional medical advice. Always seek the guidance of your qualified medical professional with questions about your health, medical condition, and product safety.